Cobalt Acetate Tetrahydrate: Unpacking a Chemical Workhorse
Historical Development
Cobalt’s status in chemistry slipped quietly into the spotlight centuries ago, with blue glass and ceramics long before anyone heard the term “rare earth.” Cobalt compounds grew up alongside industrial metal chemistry in the 19th and 20th centuries, finding their way into inks, catalysts, and coloring agents. Chemists singled out cobalt acetate—especially its tetrahydrate variant—as a reliable intermediary for advanced transitions in key industries. It didn’t pop up only because someone wanted to color porcelain. Over decades, a network of researchers and commercial labs built methods for crafting this hydrated salt, pushing industries past simple glassmaking toward fields as specialized as PET plastic manufacturing and Fischer-Tropsch catalysis. Each time oil refining or battery applications grew, another chemist likely tinkered with cobalt acetate’s crystalline blue-green compound, learning both its promise and its limits.
Product Overview
The recognizable label, “cobalt acetate tetrahydrate,” signals both heritage and flexibility. This crystalline compound grows easily and reliably under standard lab and factory conditions. Its chemical formula, Co(CH3COO)2·4H2O, doesn’t tell the full story, though. The market tracks it in shipments ranging from analytical reagent bottles in teaching labs up through metric-ton volumes for manufacturers. Quick to absorb on contact, the hydrated salt handles blending and mixing with all sorts of solvents or composite blends. Scientists and engineers pay attention to the signature blue color, the distinct vinegar-like scent, and the clumping the crystals show when exposed to open air—not just for identification, but because these properties guide every step from storage to scaling up an industrial reaction.
Physical & Chemical Properties
Shiny blue-green crystals catch the eye, but the practical handling calls for more than color checks. The tetrahydrate variety melts easily at about 140 °C and shows high solubility in water and alcohols. Toss a pinch into distilled water, and the solution turns a deep blue, a sign chemists have leaned on for years to track reaction progress or product presence. The substance weighs in with a molar mass around 249.08 g/mol. The extra four water molecules give it extra heft and help control reactivity, especially compared to the anhydrous version, which resists moisture and offers a different texture. In air, the tetrahydrate cedes water without much trouble, which means containers need a tight seal—nobody wants a batch that lost mass and ruined a mix before it even hit the reactor.
Technical Specifications & Labeling
Documentation matters—anyone who’s handled cobalt acetate knows to check purity and hydration state right away. Most suppliers publish specifications stating a cobalt content between 23–24%, with iron, copper, and nickel impurities typically held below 0.01% for advanced applications. Crystallinity, pH in solution, and water content all matter for strict QC labs. Correct labeling isn’t academic; regulations press for proper hazard codes (UN 9196, GHS pictograms for toxicity, and flammability signals). Transporters log it as a hazardous material, with specific markings for water-reactive and environmentally dangerous cargo. Failure to follow these standards doesn’t just slow things at customs—it lands fines and puts handlers at risk, especially with strict cobalt content tracking enforced in places like the EU and North America.
Preparation Method
Manufacturers use a straightforward double decomposition or neutralization reaction to get pure tetrahydrate. One common method starts with cobalt carbonate or cobalt hydroxide, reacting with acetic acid under controlled temperatures. Experienced operators know to keep the solution slightly acidic and to monitor water content: too hot or too much acid, and you’ll strip off hydration, ruining the batch’s phase and purity. The blue-green crystals precipitate naturally—no need for fussy additives. Once formed, a quick filtration removes excess solution, and drying under mild, moisture-controlled conditions locks in the hydrate. If you ever spent time in a university or plant lab, you’ll remember the sharp, unmistakable smell, the blue streaks on glassware, and the careful effort to keep every gram uncontaminated across batches.
Chemical Reactions & Modifications
This compound doesn’t just sit on the shelf—it acts as both actor and director in chemical scenes. In the lab, cobalt acetate tetrahydrate serves as a key cobalt source for exchange reactions and complex formation, especially in organometallic synthesis or the creation of catalysts. Heating pushes off water and acetic acid vapors, leaving behind oxides or anhydrous cobalt acetates, depending on conditions. Mix with strong ligands, and the cobalt swaps partners readily, forming coordination complexes used in asymmetric synthesis or catalytic cycles. These reactions don’t always go textbook-smooth; batch impurity, hydration state, or inconsistent temperature can lead to wildcards in product quality or conversion rate. Chemical engineers keep close records of reaction yields for this reason, using trace analysis to confirm nothing unexpected crept in.
Synonyms & Product Names
Search the shelves, and the same substance might appear under names like cobalt(II) acetate, cobaltous acetate, or even simply cobalt acetate (tetrahydrate). The chemical industry sometimes tags it with CAS numbers—always 6147-53-1 for the tetrahydrate. Old technical manuals and safety datasheets bounce between labels; sometimes, they refer to “Ethanol, Cobalt(2+) Salt, Tetrahydrate” or list trade names from major producers. These terms anchor the product’s long life spanning research, academia, and manufacturing. Anyone trying to source or certify a batch for technical use knows to check more than just the supplier name.
Safety & Operational Standards
Cobalt acetate tetrahydrate is helpful but never totally benign. Prolonged skin contact or inhalation can sensitize some handlers and trigger rashes or respiratory irritation. Dust control is more than a formality; facilities need proper extraction, gloves, and eye protection. Regulatory agencies treat cobalt compounds as potential carcinogens, scientifically linking long-term exposure to higher rates of lung problems. Operators undergo routine health monitoring and get annual retraining to catch any slips in safe handling. Workplaces must keep emergency wash stations accessible in every area cobalt acetate shows up—nobody wants to scramble with a spill and no backup plan. Proper disposal demands its own paperwork trail, and labs separate cobalt waste from typical chemical streams.
Application Area
Cobalt acetate tetrahydrate plays far outside simple lab demonstrations. Its top calling card lies in oxidation catalyst manufacture—especially the cobalt-manganese-bromide system that turns up in producing terephthalic acid for PET plastic. Power storage gets a boost, too, as researchers add it in lithium-ion battery electrode precursors or fuel cell catalysts. Textile and ceramics industries still depend on the pigment properties for deep, stable blue hues, especially when modern synthetic dyes can’t hold up under high heat. Some hydrocarbon manufacturers turn to cobalt acetate’s reliable reactivity for Fischer-Tropsch and hydroformylation processes, getting higher yields from synthesized fuels and specialty chemicals. You’ll even see it mixed into animal feed in micronutrient formulations when strict cobalt content is crucial for livestock health.
Research & Development
Nothing about this compound is standing still. Over the last decade, research labs began exploring cobalt acetate’s role in advanced nanomaterials—trying to stabilize nanoparticles and build new battery chemistries. Scientists and product engineers dive into the fine details of hydration state, particle morphology, and reactivity to unlock performance in next-generation batteries and high-efficiency solar cells. Some published studies point toward using tailored cobalt compounds in cancer drug delivery or antimicrobial coatings, though regulatory and toxicity hurdles slow down real-world use. Each insight sparks new projects, sometimes grinding to a halt over cost or health concerns. Still, the pace of academic publication suggests that interest isn’t slipping; the drive for better electrochemical systems and greener production methods keeps cobalt acetate in regular headlines and grant proposals.
Toxicity Research
Cobalt acetate’s toxicity profile commands respect. Toxicological data links cobalt ions to oxidative stress in biological systems, raising red flags for chronic exposure in both humans and animals. Workplace studies consistently show increased airway reactivity and even fibrotic lung changes after high airborne exposure. Animal trials reveal lower LD50 values compared to other metal acetates, underlining the need for tight exposure limits. Researchers dig into cellular mechanisms, focusing on how cobalt ions interact with DNA, mitochondria, and immune proteins. Recent findings push for bioassays on any new cobalt-based application before broad market rollout. In regions where environmental dumping led to soil or water contamination, regulators forced industry to upgrade containment and push for recycling and controlled disposal, rather than risking a buildup in groundwater or agricultural land.
Future Prospects
Looking ahead, the path for cobalt acetate tetrahydrate tracks closely with shifts in global energy and electronics. The rush for efficient battery materials favors cobalt-heavy chemistries, but worries over supply chain ethics and toxicity fuel demand for foolproof traceability and safer substitutes. Innovation in recycling cobalt compounds—pulling acetate hydrate out of waste streams for direct reuse—could slash raw material costs and environmental impact. New studies push for better understanding of nano-scale activity, targeting smarter catalysis and eco-friendly pigment formulas. Price swings will continue as markets for cobalt metal fluctuate, driven by mining, geopolitical change, and consumer electronics. Still, cobalt acetate holds a strong position in chemical supply chains, balancing performance, processability, and, with the right controls, responsible stewardship of sensitive resources.
Understanding the Importance
Cobalt acetate tetrahydrate may not show up in most people’s daily routines, but its ripple effects reach many corners of modern life. Whether in batteries, pigments, or advanced catalysts, it carries weight for industries and everyday objects. With the global push for sustainable technology, especially in energy storage, chemicals like cobalt acetate draw lots of attention. I’ve watched supply-chain teams scramble when demand for battery materials spikes, reminding me that such compounds play far more than a background role.
Batteries That Keep the World Moving
Talking about cobalt acetate usually steers the conversation toward rechargeable batteries, especially lithium-ion. You’ll find this compound in the production process for cathodes. That’s the crucial part of a battery holding the charge and releasing it, powering up everything from smartphones to electric cars. I remember the early days of electric vehicles when range anxiety and charging times frustrated almost everyone. Manufacturers turned to compounds like cobalt acetate for more reliable, long-lasting cathodes, showing its practical impact. Reports from the International Energy Agency show rising cobalt demand for electric mobility, putting a spotlight on safe, reliable supply chains.
The Lesser-Known Role in Catalysts and Chemicals
Cobalt acetate doesn’t just charge our devices. It takes center stage in chemical industry settings, acting as a catalyst to kick off reactions or speed them up. Polyester manufacturing often relies on cobalt acetate to set the reaction pace just right. Without it, you might end up with bottlenecks in production and extra waste. Years spent touring plants have shown me that process efficiency isn’t simply theory — it’s a game of survival for many businesses. The chemical’s ability to encourage oxidation reactions also matters for paint dryers and ink manufacturing.
Paints, Pigments, and a Touch of Color
Walk into any hardware store and look at the range of blues and greens on the paint wall. Artists and paint manufacturers rely on cobalt compounds for vivid colors that stay strong under sunlight. Cobalt acetate finds use in ceramics and glass too, where stability and hue matter. It’s not about luxury; it’s about providing materials that deliver both visual appeal and durability, without quick fading or safety worries.
Environmental and Health Perspectives
It’s easy to get swept up in the technical upsides, but cobalt sourcing and handling come with challenges. Reports from Amnesty International and other watchdog groups raise concerns about mining practices, prompting more companies to look for verified sourcing and recycling. I’ve spoken with battery engineers who are actively tracking every ounce of cobalt back to its origin. The chemical’s toxicity in raw form means workers and lab managers invest in good safety training and containment practices.
Rethinking the Path Forward
Technology marches forward, but so do ethical and environmental standards. Companies are investing in battery recycling, closed-loop supply chains, and alternatives that demand less cobalt without giving up performance. Universities and startups alike chase after fresh approaches because the world needs better, cleaner options. Open discussions about resources and manufacturing push everyone — from engineers to policy makers — to aim higher.
The Formula Behind Cobalt Acetate Tetrahydrate
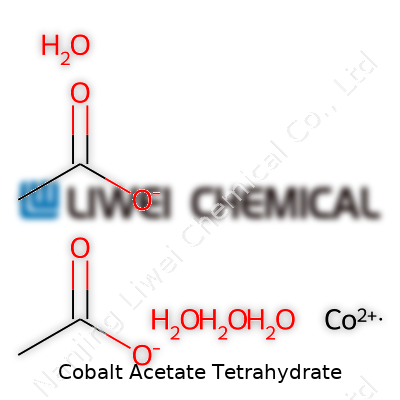
Cobalt acetate tetrahydrate holds a simple yet meaningful chemical formula: Co(CH3COO)2·4H2O. Anyone who’s handled a cobalt compound in a laboratory recognizes those familiar blue crystals. The formula tells more than just the atoms—each water molecule matters, giving the compound its unique stability and color. This hydration makes it flow differently and dissolve with ease in water, setting it apart from its anhydrous cousin.
For the molecular weight, every ion, every atom adds its share. You look at one cobalt atom (58.93 g/mol), two acetate groups (59.04 g/mol each), and four water molecules (18.02 g/mol each). Altogether, it weighs 249.08 g/mol. People in analytical labs rely on this number daily—anything less accurate could throw off entire reaction yields or research results.
Handling Cobalt Acetate Tetrahydrate in Everyday Labs
My first real taste of cobalt acetate came during an undergraduate synthesis experiment. The color struck me before anything else. A deep, vivid ruby-blue—hard to describe unless you’ve set it into a beaker. Measuring and mixing, a small error in weight translated into a failed experiment. Molecular weight wasn’t just a textbook fact; it spelled success or setback.
Research consistently points to cobalt acetate’s niche use as a catalyst. Commercial firms and university labs both count on its accurate formula and weight to create dyes, accelerate reactions, and even help grow specialty materials. People sometimes overlook the impact of impurities or varying levels of hydration, but those details set the stage for either a clean result or a lab notebook full of cross-outs.
Health, Environment, and Responsible Use
Cobalt compounds demand more than technical respect. Organizations like OSHA and the European Chemicals Agency flag cobalt salts because of respiratory and skin risks. Breathing dust or handling cobalt acetate bare-handed can harm workers, and repeated contact increases the risk. In my years working with metal compounds, I learned to mask up and glove up—no matter how familiar the material seemed.
On the environmental side, cobalt runs both hot and cold. Its role in lithium-ion batteries and catalysts means strong demand, but waste and accidental spills create contamination worries. Careless disposal leads to cobalt showing up in water and soil samples. Kids playing near industrial areas can’t tell safe dirt from contaminated soil. Researchers push for closed-loop systems and recycling, yet enforcement gaps still let waste slip through cracks.
Finding Reliable Data—and Moving Forward
Accurate chemical information underpins good science. Trusted databases—like PubChem and European Chemicals Agency dossiers—offer more than a formula and weight. They cover toxicity, handling tips, and regulatory limits. Consistently confronting up-to-date information, rather than outdated datasheets or casual supplier labels, bridges the gap between safe practice and real risk.
Better handling of chemicals starts in the classroom and ends in industry. Emphasizing correct calculations, personal protection, and thoughtful disposal sets a safer standard, both for individual workers and communities down the line. Folks who see chemicals only as numbers on a bottle miss the wider story—precision, care, and what’s best for people and the planet.
Getting Real About Risk
Cobalt Acetate Tetrahydrate has more to it than just a mouthful of a name. Working with it brings some real-world risks into play. My hands-on time in labs taught me this: The stuff can stain your skin and mess with your lungs if you slack on protection. Still, cobalt compounds support industries like batteries, dyes, and catalysts, so safe handling isn’t just a good idea—it keeps operations going without hiccups.
Controlling the Environment
Humidity ruins cobalt acetate’s shelf life in a hurry. Moisture leads to clumping and contamination. Keeping it in airtight containers, far from sinks and open windows, preserves its quality. I’ve seen coworkers toss jars into any old cabinet; the resulting mess meant lost product and extra cleanup. Mine always stayed sealed, away from heat, on a stable, labeled shelf. A workspace with reliable ventilation helps, since the dust shouldn’t end up in anyone’s lungs. Labs running regular air checks see fewer sick days. Small routines make a safer room.
Think About Containers
Glass jars work best for this kind of compound, since weak plastics might degrade and cause leaks. Each time I open a container, I check for cracks or sticky residue. Switch out compromised vessels right away. Clean up spills quickly—even a little powder can become a hazard if left on benches. Most places have spill kits, but a damp cloth and nitrile gloves handle small messes before they spread. Never skimp on clear labeling; a missing label once meant someone in my crew nearly mixed cobalt salts with food additives in a shared space. Now, our chemicals look like they walked out of a pharmacy.
Handling With Personal Protective Equipment
Anyone using cobalt acetate should glove up with nitrile or neoprene gloves. Cotton gloves can’t do the job, since powder goes straight through. Folks not wearing goggles learn fast why protection matters—one splash in your eye sticks with you. I’ve always trusted a lab coat and long pants. Good shoes stop spills from soaking in. Truth is, colleagues who laughed off PPE spent more time at the sink than on real work.
Watching Out for Others
Storing cobalt acetate shouldn’t happen next to acids, strong bases, or even food. Chemical reactions set off by a misplaced bottle won’t just make a mess—they threaten everyone nearby. Reminding new hires about chemical segregation usually saves headaches later. Listing emergency contacts and the right first aid steps near the workstation brings calm if something spills. I once watched a team freeze up until a posted cheat sheet told them exactly what to do.
What Makes This Important?
Failing to follow good handling practices means more than lost inventory; it risks the health of everyone around. The National Institute for Occupational Safety and Health rates cobalt compounds as respiratory hazards. Extended exposure links to asthma, dermatitis, or worse. I'd rather lose a few minutes to double-checking lids and gear than lose weeks to an avoidable accident.
Improvement Is Ongoing
Regular staff training cuts down on mistakes. It’s easy to let standards slip over time, especially when the pace picks up. Providing up-to-date safety sheets, and walking through a storage review every few months, keeps risks front of mind. Feedback from crew who handle cobalt acetate daily helps spot gaps in routines. Investing in good storage pays off every single day someone goes home safe.
Health Risks Linked to Cobalt Acetate Tetrahydrate
Cobalt acetate tetrahydrate isn’t something most of us meet every day, unless we walk through a lab or an industrial plant. It’s got a blue or pink crystal look, but behind the appearance, real risks need attention. Just a small amount of cobalt dust in the air can trigger reactions in people, even if the exposure happens only once. Symptoms like coughing, shortness of breath, or sore throat are quick reminders that cobalt doesn’t belong in our bodies.
Cobalt, at its core, affects more than the lungs. Over time, workers exposed to cobalt compounds see trouble with skin—redness, itching, sometimes hives. Some folks end up with asthma-like symptoms or chronic lung scarring, even after protective gear enters the scene. Other long-term effects dig deeper: cobalt has been connected to heart problems and has earned its label as a possible cancer risk by organizations like the International Agency for Research on Cancer.
Real Life Doesn’t Pause—Neither Does Risk
Spills, splashes, and dust spread easily. People who work with cobalt acetate tetrahydrate know the headache of keeping the workplace clean. If powder ends up on a bench or a lab coat, it can linger and get carried home, exposing friends and family. I remember working in a lab where glove discipline slipped just once; my hands itched and the redness stuck around for a few days. Turns out, that was one of the milder consequences.
Precaution Beats Treatment
If you touch or breathe in cobalt acetate tetrahydrate, you won’t know straight away. Symptoms often wait, or they pile on quietly, until the harm is done. Industry standards call for more than just masks and gloves. In well-run labs, staff receive training before handling any cobalt compound. Simple steps—like wiping surfaces with wet rags and running rigorous air ventilation—make a huge difference. Wash stations belong within steps of the work area. I keep a strict ‘no food, drink, or personal items’ policy wherever chemicals land.
Respirators with the right filter—the P100 or its NIOSH cousin—cut down what reaches the lungs. Protective eyewear matters just as much, since even one splash near the eye can set off discomfort and potential injury. Glove selection turns into a science. Latex by itself won’t cut it; nitrile or neoprene offers better resistance. After use, these go directly into hazardous waste bins. Open-toed shoes have no place here. Long sleeves and chemical-resistant lab coats stand as the front line of defense.
Good Practice Means Respecting the Risk
Laws and guidelines, like OSHA or EU REACH, set clear exposure limits for cobalt. Regulators have seen enough harm to lay out strict rules for ventilation and exposure monitoring. Regular blood tests check for high cobalt levels among staff in high-risk facilities. Each measure saves someone a hospital visit—or worse.
Most of us won’t ever touch cobalt acetate tetrahydrate, but the lesson here goes broader: chemicals can act invisible, but they leave a mark all the same. If safety feels like overkill, it only takes one slip to remember why we stay careful and keep learning about the materials we handle.
The Look and Make-Up of Cobalt Acetate Tetrahydrate
Cobalt acetate tetrahydrate often arrives in a lab or workshop as a reddish-pink crystalline solid. Not red like ruby, and not soft like a sunset; it’s a color easily lost in a cluttered storeroom. These crystals feel a bit chunky, sometimes like coarse salt, sometimes closer to rock candy. If you spot thin powder, something’s probably gone wrong with storage or handling, since the hydrated form tends to clump.
The amount of cobalt in each scoop or sprinkle really matters. In my time working with catalysts, I paid close attention to how much of the main metal I had, because too much junk—other metals, stray mineral bits—can throw off a reaction or mess with battery research. A bottle from a reputable source usually states “98% purity” or greater. The remaining couple percent makes room for water (the four “hydrates” in the formula), just a little sodium, or trace iron. If those impurities surf past the one percent mark, the supplier will almost always put it in the paperwork—any less and the buyer starts asking tough questions.
Where Purity Shows Its Teeth
Labs buying cobalt acetate aren’t messing around with food coloring. This stuff ends up helping make drying agents, catalysts, porcelain, pigments, and even vitamin B12. In one experiment, low-purity cobalt meant we wasted days troubleshooting a glass batch that stubbornly stayed cloudy. Turned out, a cheap batch had extra calcium mixed in, thanks to sloppy separation at the production plant.
Cobalt acetate’s real value comes from predictability. At 98% or above, the manufacturer is telling you: “You’re buying what you expect.” You can dose it by weight, trust the end product, and control contamination. It’s not just a promise—it becomes the starting line for everything downstream. If someone sends over something graded for less, the supplier should be clear about what’s been added.
Why Appearance and Purity Matter to More Than Chemists
Dirty or off-colored tetrahydrate can lead to shadowy results in electronics or glass production. When I worked alongside pottery makers, even a subtle gray tinge created by nickel meant a ruined glaze. A battery group down the hall once found excess chloride left their cells corroded. Nobody enjoys finding out the hard way.
The look of good cobalt acetate—crisp, easily recognizable crystals—keeps the process honest. Cloudy or wet-feeling batches signal it’s taking on too much moisture or breaking down. Sellers usually pack these chemicals in tightly sealed containers, sometimes with a desiccant, to keep air and water out.
Improving the Experience: From Manufacturer to User
Quality checks don’t start and end with sellers. A scientist has to crack open those containers, examine the crystals, and sometimes run a quick test or two. Over the years, color changes, odd smells, or unmarked bags have all meant picking up the phone to demand a fresh batch.
Producers like to lean on ISO certification, batch testing, and transparent certificates of analysis. These build trust and let users pick the right grade for each job. Some shops invest in in-house purity checks or partner with third-party labs to verify what’s getting poured into the beakers matches the paperwork.
Stronger communication with chemical suppliers can prevent costly slip-ups. Routine sample testing and curiosity about every label go a long way. When both sides share data—down to the nitty-gritty of elemental spectrum results—experiments tend to run smoother, and surprises become rare.
Practical Takeaways
Good cobalt acetate sticks out thanks to its sharp color, chunky texture, and purity listed right on the label. It’s more than lab supply: it’s a cornerstone of trusted scientific and industrial progress, shaped by a dependable supply line and careful inspection on the user’s end. Keeping an eagle eye on how it looks, and pushing for clarity about what’s in the bag, reduces mistakes and improves results down the line.

| Names | |
| Preferred IUPAC name | Cobalt(II) acetate tetrahydrate |
| Other names |
Cobalt(II) acetate tetrahydrate
Cobaltous acetate tetrahydrate Acetic acid, cobalt(2+) salt, tetrahydrate |
| Pronunciation | /ˈkoʊ.bɔlt ˈæs.ɪ.teɪt ˌtɛtrəˈhaɪdreɪt/ |
| Identifiers | |
| CAS Number | '6147-53-1' |
| Beilstein Reference | 1713882 |
| ChEBI | CHEBI:91247 |
| ChEMBL | CHEMBL3426574 |
| ChemSpider | 62149 |
| DrugBank | DB11276 |
| ECHA InfoCard | 100.027.277 |
| EC Number | 231-589-4 |
| Gmelin Reference | 67138 |
| KEGG | C02504 |
| MeSH | D002989 |
| PubChem CID | 6112722 |
| RTECS number | AH3325000 |
| UNII | 2O40F455MY |
| UN number | UN9197 |
| Properties | |
| Chemical formula | Co(CH₃COO)₂·4H₂O |
| Molar mass | 249.08 g/mol |
| Appearance | Pink to red crystalline powder |
| Odor | Slightly acetic |
| Density | 1.705 g/cm³ |
| Solubility in water | soluble |
| log P | -1.0 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.0 |
| Basicity (pKb) | 7.35 |
| Magnetic susceptibility (χ) | +4200e-6 cm³/mol |
| Refractive index (nD) | 1.544 |
| Dipole moment | 4.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 219.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1076.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −1743 kJ/mol |
| Pharmacology | |
| ATC code | V03AB56 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Suspected of causing genetic defects. May cause cancer. Causes damage to organs through prolonged or repeated exposure. Toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS09 |
| Signal word | Danger |
| Hazard statements | H302, H317, H319, H334, H341, H350, H360Fd, H410 |
| Precautionary statements | P210, P220, P260, P264, P273, P280, P302+P352, P308+P313, P501 |
| NFPA 704 (fire diamond) | 2-1-1 |
| Autoignition temperature | 140 °C (284 °F) |
| Lethal dose or concentration | LD50 Oral Rat 5,056 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 771 mg/kg |
| NIOSH | PC56000 |
| PEL (Permissible) | 0.1 mg/m3 |
| REL (Recommended) | 300 mg/kg |
| IDLH (Immediate danger) | IDHL: 20 mg Co/m³ |
| Related compounds | |
| Related compounds |
Cobalt(II) acetate
Cobalt(II) chloride Cobalt(II) sulfate Cobalt(II) nitrate Nickel(II) acetate Iron(II) acetate |

