Hexaphenoxycyclotriphosphazene: Challenges and Promise in Modern Industries
Historical Development
Back in the middle of the 20th century, chemists searching for better flame retardants came across cyclotriphosphazenes. At the time, widespread use of simple brominated organics exposed workers and the environment to lasting toxins. The journey to hexaphenoxycyclotriphosphazene took years, building from the first ideas about phosphorus-nitrogen cyclic compounds. Researchers noticed that replacing the chlorines on the cyclotriphosphazene ring with phenoxy groups improved both safety and flame performance. I remember watching the field grow: labs shared early data showing these materials slowed burning in plastics and textiles, hinting at something big for electronics and manufacturing. Companies worked to navigate patent hurdles and bring safer, more effective flame retardants to widespread markets.
Product Overview
Hexaphenoxycyclotriphosphazene stands out for its high phosphorus and nitrogen content, which makes it a powerful flame inhibitor, especially in epoxy resin systems and engineering plastics. Rather than boosting flame resistance with heavy metals or high-toxicity chemicals, this compound leverages its molecular design. As someone who’s spent years in materials science, I’ve seen how challenging it can be to balance fire safety with environmental and human health. Having a molecule like this in the chemist’s toolbox means safer consumer electronics, less hazardous construction equipment, and cleaner options for public transit interiors. The substance often goes by several trade names, including FR-300 and HPN-68, which sometimes confuses engineers unless they check specification sheets carefully.
Physical & Chemical Properties
The compound appears as a white to off-white crystalline powder, with a melting point around 110°C and thermal stability above 350°C. It doesn’t dissolve in water but shows good compatibility with common polymer matrices. Handling it, you notice a slightly sweet chemical odor—nothing too overpowering. It resists hydrolysis much better than early phosphazene relatives, so manufacturers don’t sweat about rapid aging or breakdown in high-humidity climates. Chemical analysis shows a molecular formula of (C6H5O)6P3N3, with a molar mass of about 693 g/mol. These properties translate to practical benefits like less volatile emission during polymer processing and fewer headaches meeting international environmental regulations.
Technical Specifications & Labeling
Industry-grade hexaphenoxycyclotriphosphazene gets shipped in multi-wall paper bags or fiber drums, usually holding 20-25 kg. Suppliers have to provide certificates of analysis, covering phosphorus and nitrogen percentages, residual chloride, particle size distribution, and loss on drying. Labs sometimes run into trouble if the material spends long periods in humid warehouses, as too much moisture bumps up loss on drying beyond spec. I’ve seen process engineers reject batches based on subtle color shifts or clumping, signaling oxidation or impurity buildup. Companies label containers with clear hazard information, including pictograms consistent with GHS requirements for irritants and environmental safety, along with recommended storage conditions: cool, dry places away from oxidizers and strong acids.
Preparation Method
Manufacturers start with hexachlorocyclotriphosphazene, reacting it with phenol in the presence of a base, typically potassium carbonate, under reflux. The phenoxy substitution needs precise temperature and pressure control so the reaction doesn’t stall or lead to unwanted side products. Solvent choice matters—toluene or other inert, high-boiling liquids help drive the reactants together. What fascinates me most here is the careful balancing act: push the reaction too hard and you get incomplete substitution, too soft and residual chloride lingers, causing discoloration down the line. Purification involves filtration, washing, and sometimes recrystallization from organic solvents. Waste handling practices have improved, with closed-loop systems reclaiming solvents and keeping releases within legal limits.
Chemical Reactions & Modifications
Hexaphenoxycyclotriphosphazene’s reactive backbone allows for further substitution if specific performance tweaks are needed. Chemists can partially replace some phenoxy groups with amino, alkoxy, or halogenated aryl units to fine-tune solubility or compatibility with particular resins. In my lab, we once experimented with mixed aryl/alkoxy systems, trading a bit of flame resistance for better mechanical synergy with certain high-performance composites. Thermal analysis reveals the compound breaks down by losing phenoxy groups and forming crosslinked phosphorus oxynitride networks—this network char acts as a shield in fire situations, slowing heat and toxic gas release. Interaction with acidic or basic environments usually passes without much drama, which keeps long-term storage and transport risks low.
Synonyms & Product Names
In catalogs, this compound pops up as HPN-68, FR-300, and sometimes just hexaphenoxyphosphazene. Chemists used to call it “hexa-phenoxy” in shorthand, which stuck in some older safety data sheets. Buyers new to the field sometimes get lost in these name shifts, so extra vigilance in verification goes a long way. Major suppliers from Europe and Asia include unique suffixes to show minor formulation tweaks or purity grades, which matters when pushing performance limits in sensitive applications.
Safety & Operational Standards
Although hexaphenoxycyclotriphosphazene rates much lower on acute toxicity compared to brominated counterparts, safe handling is essential. Operators who weigh and blend the powder wear filtered masks and gloves, especially since airborne dust irritates mucous membranes and eyes after repeated contact. I’ve seen some companies install automated dosing lines to keep worker exposure close to zero, and air monitoring shows drop-offs in personal exposure levels since switching to closed systems. Emergency guidelines recommend standard precautions—no eating or drinking in storage or blending areas, and immediate washing after contact. Disposal follows chemical waste regulations, since combustion can yield small amounts of hazardous byproducts. Data from recent inhalation studies points toward a low long-term risk, though regulatory bodies call for ongoing monitoring to address unknowns.
Application Area
Hexaphenoxycyclotriphosphazene has become an industry favorite for flame proofing printed circuit boards and insulating foams, where it keeps fires in check without spoiling the electrical properties of finished goods. Manufacturers involved in automotive and rail transport use the substance to meet strict European and American burn-through requirements, all while keeping away from halogenated systems that trigger regulatory recalls. In my field, I watched electronics giants spec hexaphenoxycyclotriphosphazene into mobile devices and smart appliances, internalizing new testing norms, fighting counterfeit ingredients, and shaving seconds off ignition times. Its physical stability in resin blends means processors avoid settling or phase separation even under harsh molding cycles.
Research & Development
Funded projects track how this flame retardant interacts with other additives, from anti-drip agents to tougheners that boost mechanical strength. I’ve seen research push toward ultra-low smoke emission and “eco-label” status so manufacturers can hit demanding green purchasing lists. Academic teams work closely with polymer scientists to decode the microscopic mechanisms behind intumescent char formation, feeding into the next generation of safer, more effective blends. Competitive pressures keep the product pipeline moving, aiming for grades that lower dosage without dropping flame-retardant ratings or making the material harder to mix. Work on biodegradable alternatives keeps the pressure high, but few challengers can boast the same blend of cost, versatility, and established production scale.
Toxicity Research
Early animal studies reported little evidence of acute poisoning or chronic health effects after inhalation or skin exposure, provided accidents were quickly addressed. Regulators pay attention to the limited breakdown byproducts and their potential impacts on aquatic organisms, leading to safety thresholds that shape legal limits for effluent and product leaching. Flame retardant residues in dust present ongoing questions about bioaccumulation, but current findings reassure public health authorities compared to legacy brominated compounds. I kept a close watch on workplace monitoring data, noting that declines in worker health complaints followed the phase-in of cyclotriphosphazene-based systems, unlike the persistent reports from halogenated alternatives. Nonetheless, environmental researchers push for more long-term studies, especially as recycling rates climb.
Future Prospects
More regions update their fire safety laws to restrict halogens and persistent organic pollutants, creating a steady demand for alternatives like hexaphenoxycyclotriphosphazene. Automakers and electronics leaders expect supply security, regulatory clarity, and ever tougher performance demands—so ongoing investment in cleaner synthesis, process automation, and green chemistry adaptation looks set to continue. Younger scientists enter the field, determined to engineer solutions that cut both cost and environmental load. Looking at new polymer matrices—biodegradable or renewable-sourced—it’s clear that further compatibility improvements will keep this molecule in the game. In the next decade, watch for big steps in circular manufacturing, with recyclers reclaiming flame retardants from scrapped goods for a second life. As the market pivots toward safer, smarter materials, those who back up lab data with real-world safety and steady supply will lead the pack.
Understanding Where It Shows Up
Hexaphenoxycyclotriphosphazene — or HPCP if you’d rather not twist your tongue — pops up everywhere that protecting people and products from fire becomes a top priority. I’ve worked with clients in electronics and furniture, and I keep running into this compound. It’s not some obscure chemical locked away in a lab. In fact, it’s widely used as a flame retardant, especially in plastics and circuit boards, tucked inside things from smartphones to home appliances.
Why Flame Retardants Like This One Matter
House fires, electrical faults, and overheated devices don’t grab headlines until something terrible happens. Statistics from the National Fire Protection Association remind me that the United States alone sees hundreds of thousands of home structure fires each year. HPCP slows the spread and lowers the intensity of fires in consumer goods, letting people escape and firefighters do their jobs more safely. I’ve read case studies that show how materials using this compound can mean the difference between a scorched area and a tragedy.
Electronics and HPCP
Printed circuit boards, the brains and nerves of modern gadgets, run hot. HPCP gets blended into epoxy resins that make up these boards. Because of its resistance to heat and chemical stability, manufacturers prefer it to older, more toxic flame retardants. RoHS (Restriction of Hazardous Substances) regulations in Europe put pressure on companies to move away from halogenated flame retardants. This shift pushes innovation and leads to safer options like HPCP. I’ve worked with hardware teams adjusting their materials list, and the switch to phosphazene-based solutions cuts regulatory headaches and environmental risk.
Beyond Electronics: Building and Transportation
I’ve seen this compound pop up in insulation foams, cable coatings, and public transport interiors. Whether it’s airplane cabin panels or train seats, HPCP makes materials harder to ignite and slower to burn. World markets, especially in Asia, have leaned on this chemistry to meet tightening safety standards without loading materials down with heavy, outdated flame retardants. In buildings, performance standards for insulating panels have climbed. HPCP makes it easier for architects and builders to deliver safe spaces without heavy compromises on weight or workability.
Concerns and Questions About Safety
No one likes discovering last year’s flame retardant now sits on a restricted list because of health or environmental links. The move away from halogen-based additives often highlights legacy damage and lingering chemical traces in household dust. Early research into phosphazene products, including HPCP, points to better environmental profiles. Still, there’s room for scrutiny on how they break down in real life. Transparent supply chains and long-term health studies help everyone make better choices.
How to Raise the Bar Even Higher
Continuous testing and tracking of chemical footprints, especially as technologies advance, mean responsible companies need to keep pushing for greener, safer alternatives that do the job. Government agencies and independent groups can encourage this progress by sharing data and keeping regulations focused on both effectiveness and public health. I’ve seen real progress come from partnerships between industry and universities, where new flame retardants often get their start before reaching mass production. Real change follows the money and the data, not just a new product name on a label.
Getting to Know Hexaphenoxycyclotriphosphazene
Hexaphenoxycyclotriphosphazene, or HPTCP, pops up in manufacturing for its flame retardant properties. It often finds its way into plastics, electronics, and even certain building materials. Industries like these lean on HPTCP for safety standards—stopping products from catching fire easily means fewer risks for both workers and end users.
Health Considerations: What the Research Shows
Safety questions matter most for folks who work closely with this chemical. Like a lot of flame retardants, the risks usually come with improper handling or lack of good protective habits. Studies show that HPTCP does not act as a mutagen or known carcinogen; toxicology data is limited, but so far there’s no evidence of severe chronic health effects from short-term exposure. Still, the dust can irritate the eyes, skin, or respiratory tract, a concern in powder-heavy workspaces.
The Environmental Protection Agency and the European Chemicals Agency classify HPTCP as low to moderate hazard when used correctly. Acute toxicity sits low on their charts, and the chemical doesn’t seem to build up inside the human body. Skin contact might spark mild irritation—think red skin or itching, similar to what you might get from fiberglass or chalky powders. The major risk comes from inhaling fine particles, which could trigger coughing or sneezing in sensitive folks.
Best Practices in Handling
Proper personal protective equipment forms the backbone of safety here. Gloves, goggles, and dust masks—simple stuff, but it works. I once toured a plastics plant using HPTCP, and air was monitored for particulates with handheld detectors. Ventilation fans cleared out stray dust, and protocols kept spills to a minimum. These steps made a difference: workers reported far fewer eye or throat problems than at other plants lacking those steps.
Training can’t be skipped. Everyone from the forklift operator to the line supervisor needs to know what to do if a sack breaks or something spills. Absorbent mats on the factory floor pick up dry powder quickly. Disposal rules stay strict. You never want someone washing leftovers down the drain or tossing chunks in the regular trash—environmental concerns make that clear.
Environmental Impact: A Bigger Picture
I’ve seen companies wrestle with the disposal question. While HPTCP doesn’t accumulate in soil or water as quickly as some older halogenated flame retardants, runoff can still carry risks for aquatic life. Regulations lean on controlled incineration, not landfill dumping. Waste management companies partner with recyclers to extract and recover useful materials, cutting down on what ends up in the ground.
The bigger story here turns on trust—workers trust that their company puts safety above shortcuts. For anyone who spends a shift near HPTCP, steps taken today shape health ten years from now. It helps to remember that safety rules exist for a reason, not just to tick off boxes but to send people home in better shape than they started.
As manufacturing keeps changing, safer alternatives keep rolling out. Whether those will fully replace HPTCP depends on price, performance, and proven safety records. Until then, companies owe everyone—inside and outside the factory—their clearest effort to use best practices and honest communication about any risks on the table.
Fire Safety for Modern Plastics
Fire-resistant materials have shaped the way we think about safety, especially in electronics and everyday consumer products. One compound earning respect in this field is hexaphenoxycyclotriphosphazene, or HPP for short. This chemical has redefined how manufacturers create safer, greener plastics for the world we live in.
Structure Fuels Performance
Hexaphenoxycyclotriphosphazene stands out because of its molecular backbone. This isn’t just chemistry jargon—its unique phosphazene ring coupled with phenoxy groups offers benefits that impact our daily surroundings. The ring provides remarkable stability, even at high temperatures. That means plastics don’t simply melt or catch fire as easily, lowering risks in laptops, car interiors, and household wiring.
Back in my university days, my lab explored several flame retardants. Many broke down quickly or gave off toxic smoke. With HPP, though, we observed a cleaner burn and less smoke. Phosphorus inside the molecule plays a significant role. During a fire, it hinders the combustion process by forming a protective char layer. This char acts like a shield, blocking oxygen and heat from fueling the flames.
Eco-Friendliness in Focus
Consumer safety doesn’t just mean fire resistance. Companies also look for options that stay away from harmful halogens. Historically, some flame retardants released toxic fumes—like dioxins—when burned. HPP contains no halogens. Recycling plastics with this compound puts less strain on ecosystems and lowers health risks for workers. The European Union, under the REACH regulation, has outlined the dangers of legacy chemicals. HPP’s chemical profile clears these environmental hurdles, earning approval for various applications, especially in places with strict regulations.
Small Additions, Strong Results
Not all flame retardants mix well with plastics. Hexaphenoxycyclotriphosphazene dissolves into most resins, including epoxy, polycarbonate, and PVC. Engineers appreciate this compatibility. Typically, only a small amount of HPP is needed to meet fire safety standards like UL 94 V-0 for electronics. Manufacturers save material, maintain the plastic’s color and toughness, and avoid unpleasant odors. Once, we tested HPP in a cable insulation blend. It didn’t cause the product to soften or crumble—a problem that plagued other fireproofing compounds.
What’s the Catch?
Properties always come with trade-offs. Some manufacturers mention HPP can be a bit pricey compared to traditional options. Supply chain hiccups may impact availability. Bulk chemical prices occasionally fluctuate, affecting production costs. Small businesses, especially those just entering the plastics market, might feel these shifts most.
Moving Toward Safer Products
Growing concern for environmental safety and health has driven research into non-toxic, efficient flame retardants. HPP’s track record—thermal protection, clean emissions, no halogens—continues to encourage further investment in greener materials. Universities, government laboratories, and private companies keep exploring how to make substances like HPP even cheaper and more effective, so wider industries can adopt them.
Public awareness and regulatory pushes matter. When designers and manufacturers choose HPP, they signal support for a future with fewer fire hazards and healthier products. People deserve to feel safe with the electronics and plastic goods they bring home. Chemicals like hexaphenoxycyclotriphosphazene help make that possible.
The Stuff Behind All Those Big Words
Hexaphenoxycyclotriphosphazene, often shortened in labs as HPTCP, plays a part in many flame-retardant processes. A mouthful of a name, but you’ll find it in insulation, electronics, plastics — the things meant to resist catching fire. Chemicals like this don’t just call for any old storage method. My time working in industrial labs hammered home the consequences if attention wavers. A slight slip-up can mean material waste at best, safety risks at worst.
Why Storage Isn’t Just a Silo or a Shelf
This compound looks like a fine, white powder — deceptively innocent. Science says it does not explode or ignite on its own. But real-world handling isn’t about resting easy with the hazard chart. Moisture is its enemy. Exposure to damp air and water starts breaking it down, changing the chemical in ways no manufacturer wants. Every barrel or container deserves protection from the tiniest crack, loose lid, or condensation.
I remember a supplier visit where bags kept in a basement started caking together. The nervous scrapes of forklifts, that smell of old cardboard and a whiff of chemical — a bad sign. In humid climates, HPTCP must live in sealed drums, preferably made of high-density polyethylene or metal with lined interiors. Containers ought to be dry before filling, and storage spaces need humidity below 60%. Not every warehouse manager checks those details, but the ones who do, save money and headaches.
Temperature and Isolation: Real Practices, Not Just the Label Rules
Simple room temperature gets mentioned in data sheets. In practice, this means 20–25°C for most operations I’ve worked with. A stable environment helps, because dramatic changes bring condensation risks. Never stack containers next to boilers, windows, or anything that gets hot. Chemical dust, if it builds up, can still irritate skin and lungs, so staff are wise to use gloves, glasses, and sometimes dust masks to keep things clean.
Storing HPTCP isn’t just about temperature; it’s about what sits nearby. Acids, bases, and oxidizers create a real problem if moisture’s in play. I’ve seen one mixing area turned into a hazmat callout because someone moved incompatible chemical drums too close together out of laziness. Having clear separation between families of chemicals makes good sense. You keep disaster at bay and make every audit smoother.
What Happens If You Ignore Good Storage?
Ignoring the basics leads to product clumping, odd odors, and a loss of performance. Manufacturers warn about shelf lives for a reason. Once packaging’s breached, companies must label opening dates and track everything closely. Mistakes drive up waste, which hits budgets and reputation. From a sustainability perspective, ruined chemicals fill up disposal bins rather than ending up in finished products.
How Better Training Could Fix Problems
Training goes further than any written checklist. Facilities with low turnover and a culture of reporting near-misses see fewer accidents. It’s not enough to trust a label or hazard code. Teams reviewing storage practices every quarter, and those using simple humidity meters, stay ahead of trouble. Companies pushing for regular safety talks get better compliance than those leaving it to a memo on the wall. So, every person who handles these barrels has a piece to play — not just for legal reasons, but for protecting both product and people.
Bringing Fire Safety to Modern Materials
Factories that produce plastics face a clear challenge: many plastic parts need some real help when it comes to fire safety. Hexaphenoxycyclotriphosphazene (HPTCP) steps in to give plastic formulas strong flame-retardant properties. This compound finds its way into the wiring insulation and plastics that get tucked behind dashboards in cars and airplanes. I have seen manufacturers scramble to meet rising safety standards, especially since reliable, non-halogenated solutions aren’t always easy to come by. Adding HPTCP brings both performance and peace of mind—something regulators and consumers both demand.
Electronics: Protecting Devices, Protecting Homes
My first touring experience through an electronics assembly plant left me with one strong impression—every device, large or small, needed rigorous fire protection. HPTCP often finds a place in printed circuit boards, connectors, and switch housings. Electronics burn risk isn’t just about material—it puts home safety on the line. Evidence from fire safety authorities underscores the need: house fires traced to faulty appliances continue to be a leading cause of home damage worldwide. Flame inhibitors like HPTCP provide a simple addition that can make the difference between a spark and a disaster.
Construction: Building Safer Spaces
Modern construction moves quickly and corners can get cut if safety tools aren’t easy to work with. HPTCP fits right into construction boards, rigid insulation foams, and even wall panels. It surprised me to learn how many buildings have hidden plastics that, unless treated, would burn fast in a fire. Builders working on public spaces need results they can trust, especially after hearing about tragic fire outbreaks in high-rises and schools. HPTCP offers a tested way to slow fire spread, adding crucial minutes for evacuation.
Textiles: Protecting Lives in Daily Use
Textiles don’t always bring to mind words like “flame retardant,” but the story changes in settings like airports, trains, and hospitals. Uniforms, curtains, and upholstered seats often contain HPTCP to meet strict safety codes. Textile producers often get caught in a balancing act—softer, more appealing fabrics need tough fire protection. Public records show stricter enforcement of flammability standards in the past decade, pushing industries to adopt safer, durable fire retardants like HPTCP.
Medical Devices: Guarding Patients and Equipment
Hospitals host countless pieces of equipment running around the clock. Electrical components, bed frames, and casings in diagnostic machines must be resistant to fire. With medical spaces, vulnerabilities put not just property but patient lives at risk. Federal requirements on medical product safety have tightened over time, and suppliers rely on additives like HPTCP to pass tough certification tests.
Challenges and Looking Ahead
Tougher regulations on environmental impacts put pressure on chemical makers. Customers want better safety with less toxicity and more recyclability. Research laboratories now chase alternatives and blends that lower environmental impact without giving up safety. I’ve seen some firms invest in recycling methods that recover flame retardants from used plastics, pointing to a future where the same compound can get reused instead of discarded. The challenge remains to balance fire safety, cost, and environmental responsibility—never an easy equation, but one where HPTCP plays a significant role for industries that can’t afford guesswork.
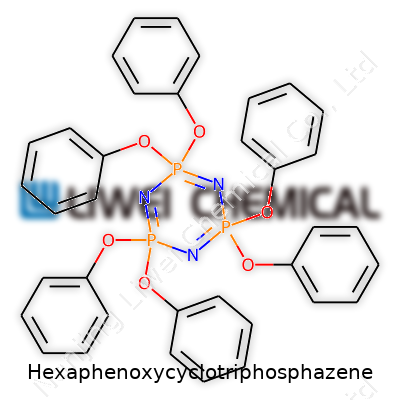

| Names | |
| Preferred IUPAC name | 2,2,4,4,6,6-hexakis(phenoxy)-1,3,5,2,4,6-trioxatriphosphazatriene |
| Other names |
HPCP
Cyclotriphosphazene hexakis(phenoxy)- Hexakis(phenoxy)cyclotriphosphazene Phenoxycyclotriphosphazene Phenoxytrimer Hexaphenoxytricyclophosphazene |
| Pronunciation | /ˌhɛks.ə.fəˌnɒk.si.saɪˌkloʊ.trɪˈfɒsfəˌzneɪn/ |
| Identifiers | |
| CAS Number | 1184-10-7 |
| Beilstein Reference | 392166 |
| ChEBI | CHEBI:39068 |
| ChEMBL | CHEMBL2105960 |
| ChemSpider | 20586559 |
| DrugBank | DB16643 |
| ECHA InfoCard | 100.027.863 |
| EC Number | 222-746-7 |
| Gmelin Reference | 84841 |
| KEGG | C14497 |
| MeSH | D017209 |
| PubChem CID | 71552 |
| RTECS number | SZ9950000 |
| UNII | Q1R1Y6Z88S |
| UN number | UN3278 |
| Properties | |
| Chemical formula | C₁₈H₁₅N₃O₆P₃ |
| Molar mass | 693.5 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.44 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.74 |
| Vapor pressure | 1.33E-5 mmHg (25°C) |
| Acidity (pKa) | 2.8 |
| Basicity (pKb) | 13.4 |
| Magnetic susceptibility (χ) | -1280.0 · 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.613 |
| Viscosity | 10-20 mPa·s (25°C) |
| Dipole moment | 3.29 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 427.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –434.5 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -7706 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H319, H335 |
| Precautionary statements | P210, P220, P221, P280, P305+P351+P338, P370+P378, P422 |
| Flash point | > 260 °C |
| Autoignition temperature | 400 °C |
| Lethal dose or concentration | LD50 (oral, rat): > 5,000 mg/kg |
| LD50 (median dose) | >5000 mg/kg (Rat, Oral) |
| PEL (Permissible) | Not established |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Trimethylolpropane phosphite
Triphenyl phosphite Hexachlorocyclotriphosphazene Phenyl dichlorophosphate Tris(2-chloroethyl) phosphate |

